Hyperactivity of the cysteine protease cathepsin S (CTSS) -either through Y132 mutations or amplification/overexpression- is a recurrent alteration in follicular lymphoma (FL) and promotes tumor growth by inducing a supportive immune microenvironment (Bararia et al, 2020). Of note, patients with CTSS-hyperactive FL had better outcomes with standard therapies, suggesting that CTSS-hyperactivity can sensitize tumors to treatment. CTSS hyperactivity has also been reported in other B cell lymphomas (BCLs) (Dheilly et al, 2020) and solid cancers (Olson & Joyce, 2015).
CTSS is mainly localized intralysosomally but can be released into the cytosol by lysosomal membrane permeabilization (LMP). Low level LMP can occur spontaneously (e.g., during cell division) and can be enhanced by treatment. Unlike other cathepsins, cytosolically released CTSS maintains its enzymatic activity at non-acidic pH. Thus, we aimed to (i) identify the determinants of the cytosolic CTSS activity, (ii) determine its impact on the regulation of apoptosis, and (iii) study LMP as a therapeutic approach for CTSS-hyperactive tumors.
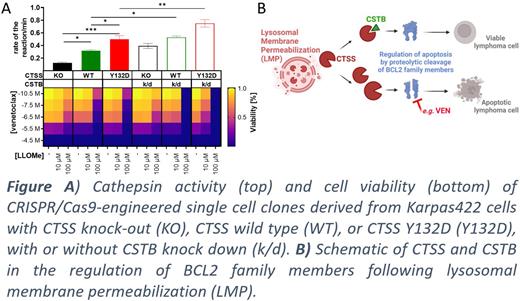
First, we accrued biochemical, functional, and clinical data supporting the role of cystatin B (CSTB) as a clinically relevant endogenous CTSS inhibitor in BCLs. Through unbiased and complementary proteomics (BioID2 labelling and co-IP followed by LC-MS/MS) we identified CSTB as a direct CTSS-interacting protein (8-fold enriched in the BCL cell line Karpas422 engineered to express CTSS wild type (WT) or Y132D vs CTSS knock-out (KO), P=0.0002). Single-cell RNA-Seq of primary FL (N=10) showed significantly higher CSTB expression in FL cells compared to normal B cells ( P=0.004). Moreover, shRNA mediated knock-down (k/d) of CSTB increased the overall cathepsin activity in BCL cell lines (N=8) by 1.5 to 5.5-fold, most notably in CTSS-hyperactive cells ( Fig A, top). We next employed LMP-inducing tool compounds (LLOMe) and clinically used drugs or analogs (desipramine, hexamethylene amiloride) to release cathepsins into the cytosol. CTSS-hyperactive Karpas422 were significantly more sensitive to LMP-inducing treatments compared to native cells (1.5 to 10-fold reduction of IC50). Importantly, CTSS hyperactivity and CSTB k/d increased LMP-mediated cell killing ( Fig A, bottom). Thus, the cytosolic CTSS/CSTB interaction determines the net cytosolic cathepsin activity and sensitivity of cells to undergo LMP-induced cell death.
Next, we hypothesized that LMP-induced cytosolic CTSS hyperactivity could prime BCLs towards apoptosis. We used BH3 profiling to functionally quantify the dependencies and interactions of BCL2 family members in BCLs with and without CTSS hyperactivity. In Karpas422 cells expressing CTSS Y132D, LMP increased overall apoptotic priming and dependencies on the anti-apoptotic proteins MCL-1 (delta priming >30 % at 10 µM, P=0.04), BCL-xL (>45 % at 10 µM, P=0.0006) and BCL2 (> 45 % at 0.5 and 1 µM, P=0.0001). We hypothesized that BCL2 family members are proteolytically cleaved by cytosolic CTSS. Indeed, e.g., BCL2 protein levels were 2.5 to 3.5-fold lower in LLOMe-treated Karpas422 cells with CTSS-hyperactivity compared to CTSS KO, and CSTB k/d further decreased BCL2 levels. To validate CTSS-mediated cleavage of BCL2, we purified FLAG-tagged BCL2 and CTSS WT and Y132D. CTSS WT efficiently cleaved BCL2 in vitro <1 hour at the top ranked predicted cleavage site and the reaction rate increased 1.3-fold for CTSS Y132D.
Finally, we hypothesized that LMP sensitizes cells to BCL2-targeting therapies ( Fig B). The combination of LLOMe-induced LMP and the BCL2 inhibitor venetoclax (VEN) showed increased cytotoxicity in CTSS-hyperactive Karpas422 cells compared to monotherapy and CSTB k/d enhanced this phenotype ( Fig A, bottom). We assessed cathepsin activities and generated dose-response curves for VEN with and without LLOMe-induced LMP in 15 primary CLL samples. Thereof, 12 samples had intermediate or high cathepsin activities and LLOMe-induced LMP increased their sensitivity to VEN, including a VEN-resistant sample in which the IC50 decreased to <3 nM.
In summary, we show that CSTB is a functionally relevant inhibitor that determines the net activity of LMP-released cytosolic CTSS. Furthermore, LMP-inducing therapies may be a promising approach to sensitize CTSS-hyperactive tumors towards apoptosis by proteolytic cleavage of BCL2 family members.
Disclosures
Oellerich:Merck KGaA: Honoraria; Roche: Honoraria; Merck KGaA: Research Funding; Gilead: Research Funding. Davids:Curio Science: Consultancy; BMS: Consultancy; BeiGene: Consultancy; Takeda: Consultancy; Novartis: Research Funding; Surface Oncology: Research Funding; Aptitude Health: Consultancy; Adaptive Biosciences: Consultancy; AstraZeneca: Consultancy, Research Funding; Ascentage Pharma: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Eli Lilly: Consultancy; Genentech: Consultancy, Research Funding; Janssen: Consultancy; Merck: Consultancy; Mingsight Pharmaceuticals: Consultancy; Research to Practice: Consultancy; Secura Bio: Consultancy; MEI Pharma: Research Funding; ONO Pharmaceuticals: Consultancy; AbbVie: Consultancy, Research Funding. Weigert:Incyte: Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal